Test Your Medical Devices at Altitude with SkyCare Air Ambulance
SkyCare.uk offers a unique opportunity for medical equipment manufacturers to trial their devices in real airborne conditions. Imagine your monitoring system, ventilator, or infusion pump operating on a live air ambulance mission at cruising altitude. With SkyCare’s dedicated air ambulance aircraft and expert clinical team, you can test, evaluate, and refine your medical devices under the exact conditions they’re built for – all while receiving professional oversight and detailed feedback.
Real Air Ambulance Testing – Authentic Altitude Conditions
There’s no substitute for the real thing. SkyCare conducts device trials aboard an operational fixed-wing air ambulance flying at altitude, rather than in a simulated lab. This means your equipment faces genuine in-flight variables such as changes in air pressure, vibration, and turbulence. You’ll discover how your device performs during take off, cruising altitude, and landing on actual missions. We often utilise return legs of repatriation flights (when no patient is being transported) to run tests, ensuring no disruption to critical patient care. It’s an authentic, high-altitude proving ground that standard ground-level testing simply can’t replicate.
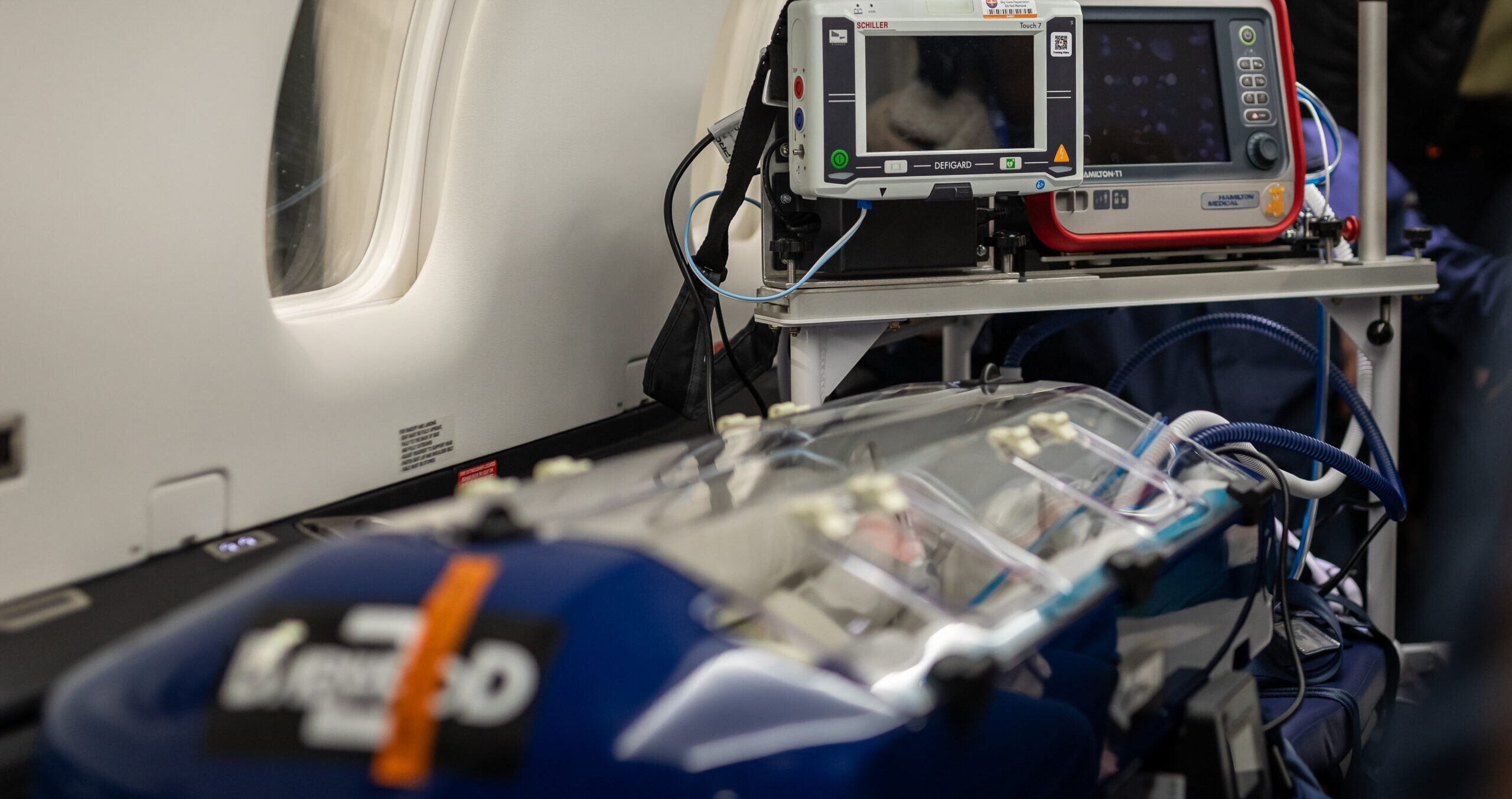
- Intensive Care in the Sky: Our aircraft functions as an ICU in the air. Your device can be integrated into a critical care scenario at 30,000 feet, verifying it works flawlessly in a pressurised cabin and noisy, moving environment.
- Paediatric & Neonatal Scenarios: SkyCare routinely transports children and infants, so we can assess devices intended for paediatric and neonatal care in real flight conditions. Whether it’s a neonatal incubator or a paediatric monitor, see how it operates where it matters most.
- Environmental Stresses: From cabin pressure fluctuations to in-flight vibrations, your product will encounter the full spectrum of stresses it might face in aeromedical service. This helps identify any performance issues early, giving you confidence that your device is truly flight-ready.
- Intensive Care in the Sky: Our aircraft functions as an ICU in the air. Your device can be integrated into a critical care scenario at 30,000 feet, verifying it works flawlessly in a pressurised cabin and noisy, moving environment.
- Paediatric & Neonatal Scenarios: SkyCare routinely transports children and infants, so we can assess devices intended for paediatric and neonatal care in real flight conditions. Whether it’s a neonatal incubator or a paediatric monitor, see how it operates where it matters most.
- Environmental Stresses: From cabin pressure fluctuations to in-flight vibrations, your product will encounter the full spectrum of stresses it might face in aeromedical service. This helps identify any performance issues early, giving you confidence that your device is truly flight-ready.
SkyCare’s in-flight trials are supervised by our medical team of NHS-affiliated research clinicians. These are doctors who are not only seasoned air ambulance practitioners but also NHS research fellows experienced in clinical trials and medical device evaluations. You’ll have expert clinical oversight at every step:
- A senior flight physician (with clinical research experience) will help plan and oversee the test protocol to ensure safety and useful data collection.
- Our clinicians can operate or observe the device in action during flight, just as a critical care doctor or flight nurse would in practice.
- Real Patient Context: If your equipment is already certified for clinical use, we have the capability (when appropriate) to use it with actual patients during missions. This means, for example, a certified monitor or ventilator could be used on a patient in flight – yielding performance data and feedback from a real case. (Patient safety remains paramount; we only do this if it’s suitable and safe for the mission.)
- Prototype Testing: If your device is a prototype or not yet certified for patient use, we can still test it onboard by having our medical staff use it on themselves or in parallel with standard equipment. For instance, a crew member might wear a vital signs sensor, or we’ll run your device alongside our regular monitors to compare readings in real time.
Clinical Oversight by NHS Research Doctors

Comprehensive Feedback and Reporting
One of SkyCare’s greatest strengths is delivering structured, insightful feedback to improve your product. After the flight, our NHS research clinicians will compile a detailed report covering all aspects of the device’s performance and usability in the air:
- Usability & Ergonomics: How easy was the device to set up and operate in a compact aircraft cabin? Could medics navigate its interface during an emergency in flight? We evaluate the user experience for clinicians working in full gear, in low-light or high-noise conditions.
- Fit & Integration: We assess how well the device fit into the air ambulance environment. Was it secure and stable during flight? Did it mount easily to our fixtures or stretcher systems? This addresses physical design considerations for aircraft use (size, weight, mounting) and compatibility with existing equipment.
- Durability: Air ambulances can experience vibrations, sudden movements, and temperature variations. We observe whether your device (and its batteries or consumables) withstands the rigors of flight without malfunctions or damage.
- Performance & Accuracy: Our team compares the device’s readings or outputs against expected values or against our own onboard equipment. For example, does your patient monitor maintain accurate vitals at altitude? Does the ventilator deliver consistent pressure and volume when cabin pressure changes? You’ll get data on how well the device performs its core function under stress.
- Reliability: We note any unexpected behavior – alarms triggered, resets, connectivity issues, etc., that occurred in-flight. Even minor glitches can become critical in the air; our report will highlight these so you can address them.
- Feedback from Clinicians: Perhaps most uniquely, you receive candid feedback from the very doctors and flight nurses who used the device. They will comment on what they liked, what could be improved, and how the device impacted patient care workflow during the mission.
This comprehensive feedback is delivered in a structured report, ideal for your R&D and quality teams. It can help guide refinements, support your regulatory submissions with real-world use data, or even serve as a marketing testament that your device has been “air ambulance tested.”
Broad Range of Devices and Use Cases Welcome
We invite manufacturers of all types of medical equipment to take advantage of this service. Whether your product is designed for hospital ICUs, ambulances, or specifically for air medical transport, we can put it through its paces in our unique setting. Examples of devices that can be tested at altitude with SkyCare include:
- Patient Monitoring Systems: Multi-parameter monitors, portable ECG/EKG devices, pulse oximeters, neonatal monitors – ensure they stay accurate at 30,000 feet.
- Ventilators & Respiratory Devices: Transport ventilators, CPAP/BiPAP machines, oxygen concentrators – see how pressure changes affect performance and oxygen delivery.
- Infusion Pumps & Syringe Drivers: Test infusion devices for consistency and alarm behavior during turbulence and varying altitudes.
- Diagnostic Tools: Portable ultrasound machines, blood analyzers, or any point-of-care diagnostic equipment – find out if they function reliably in-flight.
- Innovative Prototypes: Have a cutting-edge idea like a new wearable vital sensor or telemedicine device for medevac use? Try it in the real environment it’s meant for and gather invaluable data before full market launch.
SkyCare is especially keen to collaborate with companies developing equipment for aeromedical and critical care transport. If your device is intended for EMS helicopters, fixed-wing ambulances, or other transport settings, testing with us will validate it in the exact scenario it’s built to serve. Even if your product is early in development, an altitude trial can reveal insights that shape your design to better meet the needs of flight medics and patients.

Our Two-Fold Advantage: Real Flight + Expert Medicine
1. A Real Air Ambulance at Altitude: SkyCare provides the aircraft, crew, and true flight conditions needed to test your device properly. You’re not in a simulator or ground lab – you’re aboard an active air ambulance mission. This is a one-of-a-kind laboratory in the sky, where your device can be evaluated during actual aeromedical operations. The authenticity of this environment gives you confidence that any results and data are directly applicable to real-world use. There’s simply no better way to prove your device’s airworthiness and robustness than by flying it on our missions.
2. Clinical Oversight from NHS Research Doctors: Unlike standard engineering tests, our program is driven by clinicians who understand both patient care and research methodology. SkyCare’s doctors are NHS-trained, with roles as research fellows, meaning they have experience in conducting clinical trials and medical studies. Their involvement ensures that the testing process is safe, ethically sound, and yields medically relevant insights. You benefit from their dual perspective as front-line healthcare providers and scientific observers. They’ll not only keep your trial aligned with clinical best practices but also translate in-flight observations into meaningful recommendations for your design and user training. This marriage of aviation and medicine is what makes SkyCare’s offering truly exceptional.

Flexible Testing Engagements (Paid or Trade Options)
We understand that innovation thrives on collaboration, so we offer flexible ways to partner with SkyCare for your in-flight testing needs. Manufacturers can engage with us through a standard paid testing service or explore trade arrangements to suit both parties. For example, a trade arrangement might involve providing SkyCare with your equipment or other support in exchange for the testing opportunity and feedback. We are open to creative collaborations – the goal is to make altitude testing accessible and mutually beneficial.
What you can expect from a SkyCare testing partnership:
- Planning & Logistics: Our team will work with you to understand your device, testing objectives, and any specific requirements. We’ll discuss logistics such as scheduling a suitable flight (typically aligning with a repatriation return leg or a dedicated test flight), shipping or handling of the equipment, and any training your device might require for our medics.
- In-Flight Test Execution: We integrate the device into the flight. Depending on the plan, this could be during an actual patient transport (for certified devices) or a non-patient flight scenario. Our crew will operate the device according to your instructions and predefined test protocols, ensuring we gather the data points you need (performance metrics, user observations, etc.).
- Post-Flight Debrief & Report: After the flight, we conduct a debrief with our clinical team and compile the detailed feedback report. We can also arrange a discussion session with your engineers or product team to walk through the findings, answer questions, and brainstorm solutions for any issues observed.
- Follow-Up: Should you wish to refine your device and test again, or try it in a different scenario (for example, after modifications or in another use case like pediatric vs adult patient), we are enthusiastic to continue the collaboration. Our relationship can be one-off or ongoing, based on your development cycle.
Whether you choose a paid service model or a trade partnership, rest assured that SkyCare is committed to delivering value far beyond a typical test report – we see ourselves as an extension of your R&D team, helping drive your innovation to new heights (literally!).

Ready to Take Off? Get in Touch
SkyCare’s altitude testing service is a one-of-a-kind opportunity to validate and showcase your medical device’s performance in the air. It’s a chance to differentiate your product with real-world proof that it can handle the demands of aeromedical care. We make the process professional, safe, and tailored to your needs, all while maintaining an approachable, collaborative attitude.
Interested in learning more or scheduling a test flight for your equipment? Contact us today to discuss the next steps. Our team will happily talk through the logistics, scheduling, and desired outcomes to craft a testing plan that works for you. Together, let’s ensure your medical device reaches its full potential in any environment – ground or sky.
Reach out to SkyCare’s team to elevate your device testing program – literally. We look forward to partnering with you and helping your innovation soar.

Feedback
What our clients say.
Accreditations
AOC (Oriens Flight Operations) #2480

British Business & General Aviation Association


 SkyCare
SkyCare